Introduction
In the world of biotechnology and life sciences, few cell lines have had as much impact as Chinese Hamster Ovary (CHO) cells. Among their various derivatives, CHO-K1 cells have emerged as one of the most widely used and trusted workhorses for scientific research and industrial applications. From their humble beginnings as cells isolated from the ovary of a Chinese hamster in the 1950s to their current role as the foundation of modern biologics production, CHO-K1 cells continue to revolutionize drug discovery, therapeutic protein manufacturing, and fundamental cell biology.
Definition
Chinese Hamster Ovary (CHO) K1 cells are a widely used immortalized mammalian cell line originally derived from the ovary of the Chinese hamster (Cricetulus griseus). The K1 subline was isolated for its strong growth characteristics and genetic stability, making it one of the most common variants for research. CHO-K1 cells are adherent, easy to culture, and highly adaptable to genetic manipulation, which has led to their extensive use in biotechnology for recombinant protein production, gene expression studies, and drug development.
Origins of CHO Cells
The CHO cell line was first derived in 1957 by Dr. Theodore T. Puck and colleagues at the University of Colorado. Originally, the cells were isolated from the ovary of a Chinese hamster (Cricetulus griseus). Their establishment represented a breakthrough in mammalian cell culture because the cells were relatively easy to grow, adapt to different conditions, and maintain in laboratories.
Over the decades, researchers generated multiple derivatives of the parental CHO line, including CHO-K1, CHO-DG44, and CHO-S. Each has unique characteristics that make it suitable for specific applications. The K1 strain, in particular, was cloned from a single cell in 1958 and has since become one of the most reliable models for molecular biology and industrial protein production.
Biological Characteristics of CHO-K1 Cells
CHO-K1 cells are epithelial-like adherent cells, meaning they typically grow attached to surfaces in culture. However, through adaptation, they can also be grown in suspension, which is essential for large-scale industrial applications.
Key features include:
- Stable karyotype with genetic flexibility: While CHO cells exhibit some chromosomal abnormalities (aneuploidy), this actually enhances their adaptability, allowing genetic modifications and recombinant protein expression with fewer complications compared to human cell lines.
- Rapid growth: CHO-K1 cells double quickly, making them efficient for both laboratory experiments and large-scale bioproduction.
- Adaptability to serum-free media: This property is vital for pharmaceutical applications, as serum-free culture reduces contamination risks and simplifies downstream purification.
- Tolerance to genetic engineering: CHO-K1 cells can accept and express foreign DNA efficiently, enabling researchers to insert genes that code for therapeutic proteins.
Why CHO-K1 Cells Are So Widely Used
Several factors explain the enduring dominance of CHO-K1 cells in research and biomanufacturing:
Safety for human use:
Since CHO cells are non-human and non-tumorigenic, they are considered safe for producing therapeutic proteins. Regulatory agencies like the FDA have decades of familiarity with CHO-derived products.
Ability to perform post-translational modifications:
Proteins produced in CHO-K1 cells undergo essential modifications – such as glycosylation, folding, and assembly – closely mimicking those that occur in human cells. This makes them far superior to bacterial expression systems like E. coli for producing complex biologics.
Consistency and scalability:
CHO-K1 cultures can be scaled from small laboratory flasks to massive bioreactors containing thousands of liters, while maintaining consistent protein quality.
Versatility in application:
Beyond protein production, CHO-K1 cells are valuable for genetic studies, drug toxicity testing, gene editing research, and basic cell biology.
CHO-K1 in Biopharmaceutical Production
Perhaps the most significant contribution of CHO-K1 cells is their role in biopharmaceutical manufacturing. Today, the majority of FDA-approved therapeutic antibodies and recombinant proteins are produced in CHO cells. Examples include monoclonal antibodies used to treat cancers, autoimmune diseases, and infectious diseases.
Monoclonal Antibodies:
CHO-K1 cells are the primary choice for generating monoclonal antibodies due to their ability to produce high yields of properly folded and glycosylated proteins. These antibodies are crucial in targeted therapies, where they bind specifically to disease-associated molecules.
Recombinant Proteins:
In addition to antibodies, CHO-K1 cells produce hormones, clotting factors, enzymes, and growth factors. For instance, tissue plasminogen activator (tPA), a life-saving drug for stroke patients, was among the first recombinant proteins produced in CHO cells.
Biosimilars and Next-Generation Biologics:
With many blockbuster biologics reaching the end of their patent life, CHO-K1 cells are now central to the development of biosimilars – cheaper but equally effective versions of existing biologics. Moreover, ongoing innovations in cell line engineering are enabling the production of next-generation therapeutics, including bispecific antibodies and fusion proteins.
Research Applications of CHO-K1 Cells
Beyond industrial protein production, CHO-K1 cells serve as a versatile model system in academic and clinical research.
Toxicology and Drug Screening:
CHO-K1 cells are frequently used to test the cytotoxicity of new compounds. Their robust growth and well-characterized genome make them a reliable model for predicting drug responses.
Genetic Studies:
Scientists have used CHO-K1 cells to study DNA repair pathways, chromosomal stability, and gene expression mechanisms. The availability of genome editing tools like CRISPR-Cas9 has further expanded their utility.
Cell Biology Research:
CHO-K1 cells have been instrumental in uncovering mechanisms of protein trafficking, receptor signaling, and metabolic regulation.
Vaccine Development:
In some cases, CHO cells are used for producing viral proteins or virus-like particles that can be developed into vaccines.
Recent Advances in CHO-K1 Cell Engineering
Modern biotechnology has taken CHO-K1 cells beyond their natural capabilities through advanced engineering techniques:
- CRISPR-Cas9 genome editing: Allows precise modification of genes to enhance productivity, improve glycosylation patterns, or reduce undesired byproducts.
- Metabolic engineering: Researchers are reprogramming cellular metabolism to increase yields and reduce waste accumulation.
- Omics technologies: Genomics, proteomics, and transcriptomics studies are helping scientists better understand CHO-K1 biology, leading to rational improvements in cell line development.
- Artificial intelligence and machine learning: These tools are increasingly applied to optimize culture conditions and predict production outcomes, further enhancing the efficiency of CHO-based systems.
Challenges and Limitations
Despite their immense utility, CHO-K1 cells are not without limitations:
- Non-human glycosylation: While CHO cells perform glycosylation similar to humans, subtle differences can sometimes affect protein efficacy or immunogenicity.
- High production costs: Large-scale bioreactors, specialized media, and purification processes contribute to the high price of CHO-derived biologics.
- Genetic instability: Over time, CHO-K1 cells can accumulate genetic changes, leading to variability in protein production and requiring careful monitoring.
Researchers are actively addressing these challenges through improved culture systems, synthetic biology approaches, and next-generation host cell lines.
The Future of CHO-K1 Cells
Even as alternative expression systems – such as human cell lines, yeast, or plant-based platforms – gain traction, CHO-K1 cells are unlikely to be replaced soon. Their long track record of safety, regulatory acceptance, and adaptability ensures they will remain central to biologics manufacturing.
Future developments are likely to focus on:
- Enhancing productivity to reduce drug costs.
- Engineering human-like glycosylation pathways for improved therapeutic compatibility.
- Developing fully defined, serum-free, animal-component-free media for sustainable production.
- Integrating automation, robotics, and AI to streamline CHO-K1 cell-based bioprocesses.
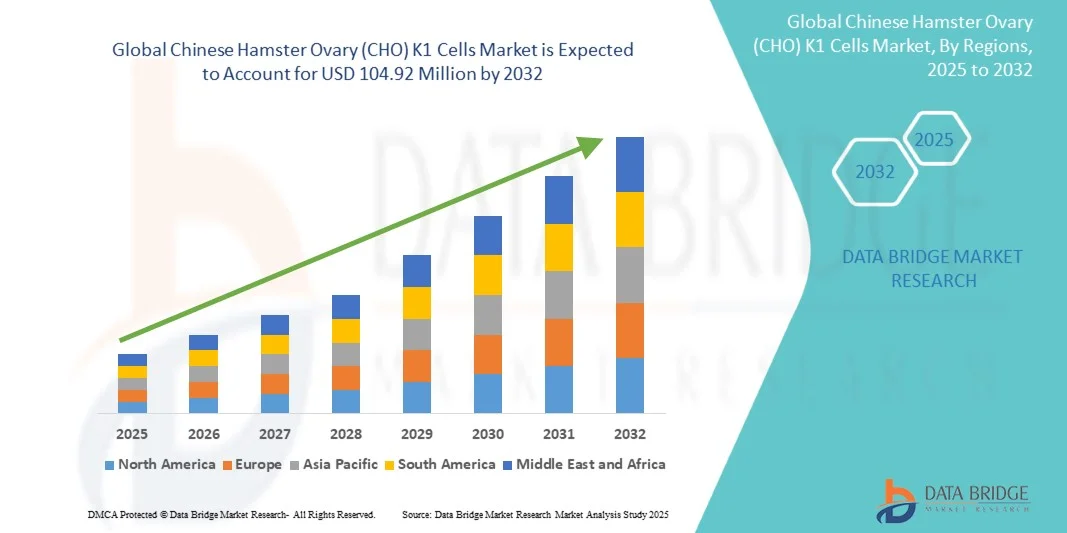
Growth Rate of Chinese Hamster Ovary (CHO) K1 Cells Market
According to Data Bridge Market Research, the Chinese Hamster Ovary (CHO) K1 Cells market is projected to grow at a compound annual growth rate (CAGR) of 8.85% from its 2024 valuation of USD 53.24 million to USD 104.92 million by 2032.
Learn More: https://www.databridgemarketresearch.com/reports/global-chinese-hamster-ovary-cho-k1-cells-market
Conclusion
From their discovery over half a century ago to their role as the backbone of today’s biopharmaceutical industry. CHO-K1 cells have transformed medicine and biotechnology. Their ability to reliably produce complex therapeutic proteins, combined with their adaptability to modern genetic and process engineering, ensures their relevance well into the future.