Article:
The Retinitis Pigmentosa (RP) Treatment Market is experiencing significant momentum as research efforts and technological advancements continue to transform the landscape of retinal degenerative disease management. Retinitis pigmentosa is a group of rare, inherited eye diseases that cause progressive vision loss due to the deterioration of photoreceptor cells in the retina. Affecting approximately 1 in 4,000 people globally, RP has long posed a clinical challenge due to the limited treatment options available. However, recent developments in gene therapy, stem cell therapy, and retinal implants have opened promising avenues for affected individuals.
Market Overview
The global retinitis pigmentosa treatment market is being fueled by growing awareness, increased research funding, and the emergence of novel therapeutics. The market size is expected to witness robust growth over the next decade, driven by both government and private sector investments in rare and orphan diseases.
Key Drivers
- Advancements in Gene Therapy:
The approval of gene therapies such as Luxturna (voretigene neparvovec) has paved the way for precision treatments targeting specific genetic mutations causing RP. Clinical trials for other gene-editing tools, including CRISPR-Cas9, are also progressing rapidly. - Stem Cell Research:
Stem cell-based therapies offer potential for retinal regeneration, aiming to restore damaged photoreceptor cells. Ongoing trials and collaborations between biotech firms and academic institutes are expanding this frontier. - Retinal Implants and Bionic Eyes:
Technological innovations such as the Argus II retinal prosthesis system have shown that electronic implants can partially restore visual function in RP patients. Further development in microelectronics and AI is enhancing these outcomes. - Increased Orphan Drug Designations:
Many RP treatments are now receiving orphan drug status, providing regulatory support and incentives for pharmaceutical companies to invest in development. - Rising Prevalence and Awareness:
Enhanced diagnostic techniques and public awareness campaigns are contributing to earlier detection and intervention, thereby expanding the potential patient pool for new therapies.
Challenges
Despite encouraging advancements, the RP treatment market faces several obstacles, including:
- High cost of gene and cell therapies.
- Limited access in low- and middle-income countries.
- Regulatory hurdles and long clinical trial durations.
Get More Details:
https://www.databridgemarketresearch.com/reports/global-retinitis-pigmentosa-treatment-market
Regional Insights
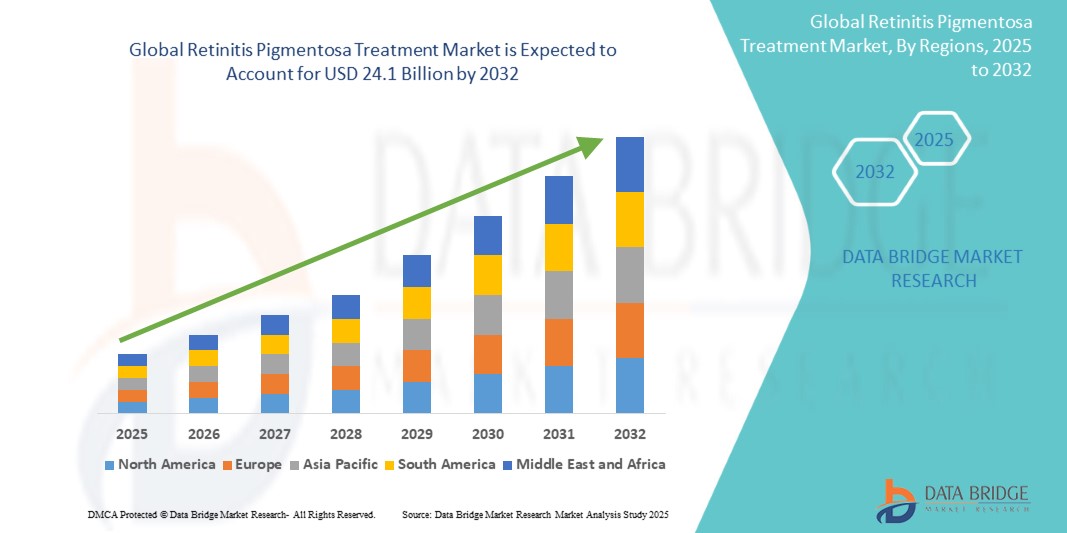
North America and Europe dominate the market due to strong research infrastructure, funding support, and the presence of major pharmaceutical players. Meanwhile, Asia-Pacific is poised for faster growth, spurred by increasing healthcare expenditure and growing interest in ophthalmic research.
Future Outlook
The next decade holds substantial promise for the RP treatment market. As more targeted therapies gain approval and novel drug delivery systems are introduced, patient outcomes are expected to improve significantly. Collaborative efforts between biotech firms, research institutions, and healthcare providers will be critical in turning these breakthroughs into accessible treatments.